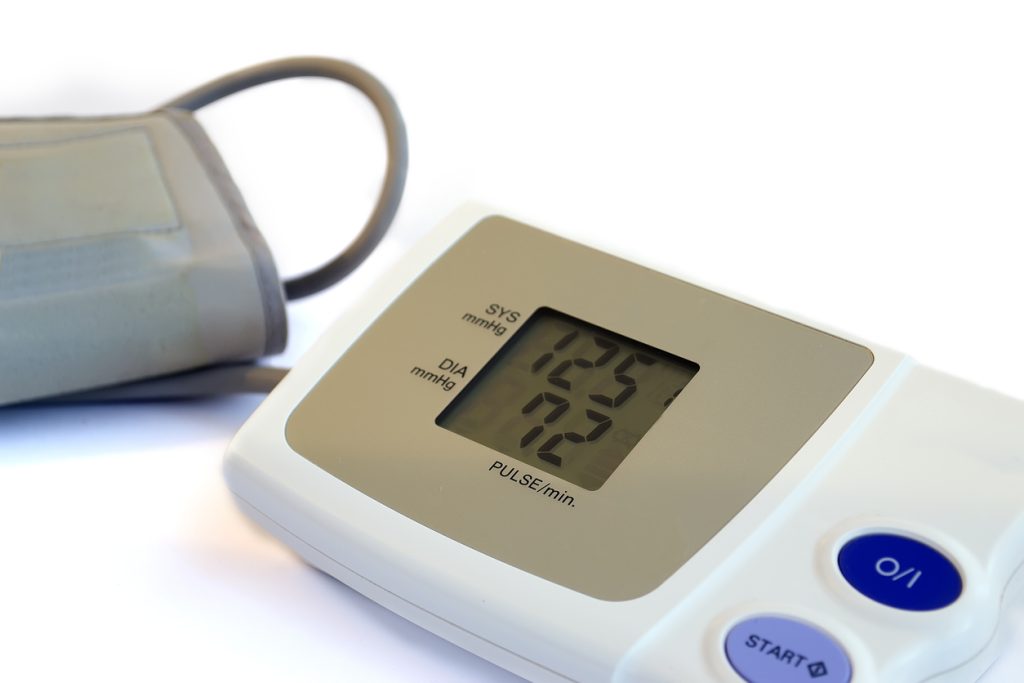
SS 620 is a standard that sets out the requirements for importers and wholesalers to develop a quality management system (QMS) that conforms to the requirements of the good distribution practice for medical devices (GDPMDS). It ensures that organizations dealing with medical devices have quality management systems in place to maintain device quality throughout the distribution process.
Certification
SS 620 Good Distribution Practice for Medical Devices
Share This Post
Share on facebook
Share on linkedin
Share on twitter
Share on email
About
Benefits
SS 620 GDPMDS QMS will help you:
- Manage and control the medical devices appropriately throughout the supply chain, ensuring their safety and performance at the point of use.
- Assure stakeholders that the organization can maintain quality, safety, and performance of medical devices while under its custody.
- Fulfill establishment licensing requirements.
The formula to a more productive organisation with better and happier employees lies in your decision making.
Certification of management system serves as excellent marketing tools, enhancing brand recognition, and instilling greater confidence among your business partners and end customers.
 Globally, GICGâs logo is widely recognised and respected as a trusted symbol of quality, safety and sustainability.
